With the surge of electric vehicles (EVs), development of low-cost, high energy density batteries is a necessity. High energy density cathodes have garnered tremendous interest as they hold the key to high energy density lithium-ion batteries which are needed to overcome ‘range anxiety’ that is holding back universal uptake of EVs. Scientists and engineers from academia, government labs and industry have been working together to overcome technical challenges and limitations in the quest to achieve the optimal combination of battery cost and performance.
High-Ni cathodes are popular owing to their high energy density and good rate capability.
Global leaders in the battery field are working to further enhance the performance of high-Ni cathode materials as well as on the development of novel Ni-based cathode materials. With all the ongoing exciting research endeavors, together they see a bright future for Ni-based cathodes.
Ni-rich cathodes, associated challenges, and strategies
Among 3d transition metals, nickel ensures higher cell voltage and a continuous voltage profile, as well as a delocalized electron density i.e., good electronic conductivity. By employing higher amounts of Ni in the cathodes, the capacity increases. But this comes with a caveat - the adverse impact on the thermal stability and long-term cycling performance. Residual Li (Lithium hydroxide and lithium carbonate) form on the surface of a Ni-rich compound over time when exposed to air, which is detrimental to electrode slurry making and cyclability.
Scientists have come up with different strategies for solving the issues encountered in high-Ni-containing cathodes. Bulk doping (introducing impurities) as well as surface stabilization techniques impede gas evolution and other issues.

Dr. Lin's group at Virginia Tech has screened a variety of doped chemistries and noticed that there exists a preference for whether the dopant accumulates in the surface or resides in the bulk. They demonstrated that Ti (titanium) doping in the cathode locks oxygen into the lattice quite nicely owing to the stronger bonding energy between Ti and oxygen. Comparatively, W (tungsten) doping yields the same outcome, but such oxygen retention is weaker in the case of Mn (manganese) doping.
Surface accumulating dopants protect the cathode surface against side reactions with the electrolyte, while bulk residing dopants could stabilize the structure through mitigating phase transitions.
By taking advantage of this preferential behavior for dopant distribution, both the surface and bulk of the cathode can be stabilized by simultaneous doping. One such example is Mg/Ti co-doped LiNiO2.
Dr. Lin’s group is also working on enhancing capacity utilization percentage. Their strategy is to ensure each grain has the same charging pattern in polycrystalline high-Ni containing layered oxides. They have found out that the grain crystallographic orientation dictates the charging behavior and mechanical properties of Ni-rich layered cathodes.

Meanwhile, Dr. Manthiram’s group at University of Texas at Austin has been extensively working on the development of high Ni cathodes and has gone from 94% Ni and 6% Co (cobalt) in their cathode to a Co-free composition. They demonstrated that the performance integrity can be preserved without Co. One such example attained by their efforts is the high‐nickel NMA (LiNi1-x-yMnxAlyO2) which is a cobalt‐free alternative to NMC and NCA cathodes for lithium‐ion batteries.
They performed a comparative study on various high nickel-containing cathode compositions: NMA-89, NMC-89, NCA-89, and Al-Mg co-doped NMC (NMCAM-89) where 89 refers to 89% nickel content.
The corresponding initial discharge capacities were 216, 226, 220, 213 mAh g-1, respectively. NMA-89 exhibits a higher average operating voltage vis a vis NMC-89 despite the lower capacity.
One other strategy is employing single crystals. With a single crystal, the major advantage is that you can reduce surface activity; it may also reduce residual lithium. For example, Dr. Dahn’s group at Dalhousie University took SEM (scanning electron microscopy) images of cross-sections of cathode particles from single-crystal NMC 811 after 1100 cycles, and they showed no sign of cracking which is a testimony to the efficacy of the single-crystal strategy.

New classes of high Ni-containing cathodes
One approach for addressing current limitations is the development of new cathode materials on the basis of known Ni chemistries.
LiNixFeyAlzO2 cathode material
In the quest for desirable electrode materials, researchers from Oak Ridge National Laboratory, USA have developed a new class of nickel-rich layered cathodes for batteries. This new material is comprised of lithium, nickel, iron, aluminum, and oxygen with the general formula LiNixFeyAlzO2 (x + y + z = 1) (x ≥ 0.8, moving to 90% nickel as the project progresses) abbreviated as NFA. Al and Fe have very similar sizes and have similar sizes as Ni3+ so they can substitute very easily and cleanly in the transition metal layer.
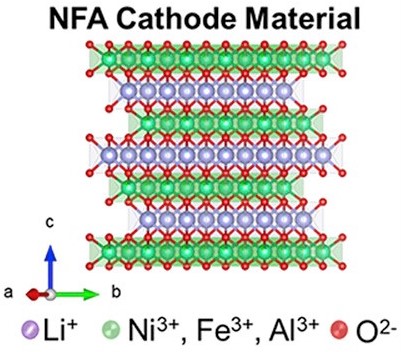
NFA is synthesized by the co-precipitation method in continuous stirred-tank reactors (CSTR). Given the similarities in the ionic radii of Li+ and Ni2+ ions, cation mixing is a potential challenge in Ni-rich cathodes which can result in ion migration bottlenecks leading to capacity loss. NPD (Neutron Powder Diffraction) refinements indicated only ≈4% Li and Ni antisite defects for the synthesized NFA compositional variants which is similar to that observed for conventional cobalt-based NMC-type materials.
Introducing tiny amounts of Al (Aluminium) and Fe (iron) improves structural stability as well as safety. The results for these promising cathodes are highlighted in two publications. NFA has a layered structure with the same space group as NCA cathode material ( ). Moreover, specific capacities (~200 mAh g-1) and voltage window of NFA materials are like those of NCA and NCM-811. In the compositional space explored, LiNi0.8Fe0.05Al0.15O2 demonstrated reasonable rate capability and cycling stability with 80% capacity retention after 100 charge/discharge cycles. Coatings such as Zirconia and phosphates are being studied to protect the Ni-rich NFA cathodes against parasitic reactions.
Conclusion
High capacity, high energy density materials are essential to fulfill long-distance travel. For the US and Canada as well as some countries in Europe, energy density is the most dominant factor in take up of electric vehicles. Research is ongoing for finding high nickel cathodes with a combination of the best cyclability, and best thermodynamic stability. Ni-based cathodes are already well established from a commercialization standpoint and significant progress is being made with regard to the reliability of the Ni-rich chemistry which is being successfully adopted by OEMs. Tesla has been using Ni-rich chemistries e.g., NCA for some time now and in the next two years, several companies plan to release lithium-ion batteries that employ cathodes with greater than 90% nickel.