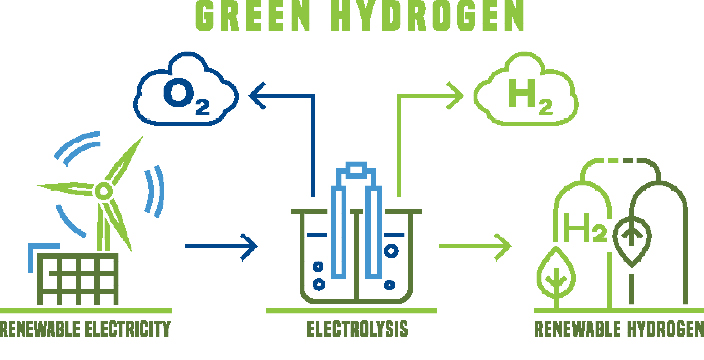
Hydrogen is 14 times lighter than air, non-toxic, colourless and odourless, and burns without residue with a colourless flame. It is gaseous down to -253 °C, after which it liquefies. It is a very reactive element that only occurs in a bound form, for example as a hydrogen molecule, in water with oxygen or in methane with carbon.
Hydrogen itself is energy intensive to produce. Worldwide annually, 30 million tonnes of “grey” hydrogen are produced from fossil fuels such as natural gas or oil, mostly by steam reforming. This is a process that converts water and methane into hydrogen and carbon dioxide (CO2). And every tonne of hydrogen produces ten tonnes of CO2.