The major routes of nickel exposure that have toxicological relevance to the workplace are inhalation and dermal exposures. Oral exposures can also occur (e.g., hand to mouth contact), but the institution of good industrial hygiene practices (e.g., washing hands before eating) can greatly help to minimize such exposures. Therefore, this chapter mainly focuses on the target systems affected by the former routes (i.e., the respiratory system and the skin). To the extent that other routes (such as oral exposures) may play a role in the overall toxicity of nickel and its compounds, these routes are also briefly mentioned. Focus is on the individual nickel species most relevant to the workplace, namely, metallic nickel and nickel alloys, oxidic, sulfidic and soluble nickel compounds, and nickel carbonyl.
4.1 METALLIC NICKEL
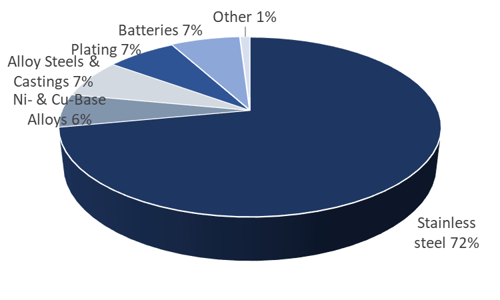
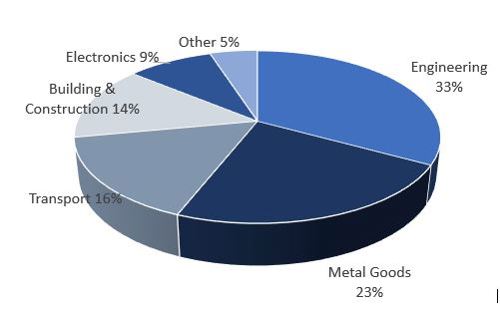
Occupational exposure to metallic nickel can occur through a variety of sources. Most notable of these sources are metallurgical operations, including stainless steel manufacturing, nickel alloy production, and related powder metallurgy operations. Other sources of potential occupational exposure to metallic nickel include nickel-cadmium battery manufacturing, chemical and catalyst production, plating, and miscellaneous applications such as coin production. In nearly all cases, metallic nickel exposures include concomitant exposures to other nickel compounds (most notably oxidic nickel, but other nickel compounds as well), and can be confounded with exposure to other non-nickel substances specific to the particular activity or process executed in the workplace. Therefore, it is important to summarize those health effects which can most reasonably and reliably be considered relevant to metallic nickel in occupational settings, even though other nickel and non-nickel compounds may be present.
4.1.1 Inhalation Exposure: Metallic Nickel
With respect to inhalation, the only significant health effects seen in workers occupationally exposed to metallic nickel occur in the respiratory system. Based on the toxicological information available from nickel compounds, the two potential effects of greatest concern with respect to metallic nickel exposures would be non-malignant respiratory effects (including asthma and fibrosis) and respiratory cancer. Factors that can influence these effects include: the presence of particles on the bronchio-alveolar surface of lung tissue, mechanisms of lung clearance (dependent on solubility), mechanisms of cellular uptake (dependent on particle size, particle surface area, particle charge) and the release of Ni (II) ion to the target tissue (of importance to both carcinogenicity and Type I immune reactions leading to asthma).
In the case of respiratory cancer, studies of past exposures and cancer mortality reveal that respiratory tumors have not been consistently associated with all chemical species of nickel. Metallic nickel is one of the species for which this is true. Indeed, epidemiological data generally indicate that metallic nickel is not carcinogenic to humans. Over 40,000 workers from various nickel-using industry sectors (nickel alloy manufacturing, stainless steel manufacturing, and the manufacturing of barrier material for use in uranium enrichment) have been examined for evidence of carcinogenic risk due to exposure to metallic nickel and, in most instances, accompanying oxidic nickel compounds and nickel alloys [14, 15, 85-88]. No nickel-related excess respiratory cancer risks have been found in any of these workers.
Of particular importance are the studies of Cragle et al. [88] and Arena et al. [14]. The former study of 813 barrier manufacturing workers is important because of what it reveals specifically about metallic nickel. There was no evidence of excess respiratory cancer risks in this group of workers exposed predominantly to metallic nickel. The latter study is important because of its size (>31,000 nickel alloy workers) and, hence, its power to detect increased respiratory cancer risks. Exposures in these workers were mainly to oxidic and metallic nickel. Only a very modest relative risk of lung cancer (RR, 1.13; 95% CI 1.05-1.21) was seen in these workers when compared to the overall U.S. population (smoking not accounted for) and the risks decreased and became statistically nonsignificant (RR, 1.02; 95% CI 0.96-1.10) in comparison to local populations. The lack of a significant excess risk of lung cancer relative to local populations, combined with a lack of an observed dose response with duration of employment regardless of the comparison population used, suggests that other non-occupational factors associated with geographic residence or cigarette smoking may explain the modest elevation of lung cancer risk observed in this cohort [14].
While occupational exposures to metallic nickel in the nickel-using industry have historically been low (< 0.5 mg Ni/m3), certain subgroups of workers, such as in powder metallurgy, have been exposed to higher concentrations of metallic nickel (around 1.5 mg Ni/m3) [14]. Such subgroups, albeit small in size, have shown no nickel-related excess cancer risks.
In studies of nickel-producing workers (over 6,000 workers) where exposures to metallic nickel have, in certain instances, greatly exceeded those found in the nickel-using industry, evidence of a consistent association between metallic nickel and respiratory cancer is lacking. For one of these cohorts, the International Committee on Nickel Carcinogenesis in Man [24] did not find an association between excess mortality risk for respiratory cancers and metallic nickel workers, whereas another group of researchers [89] found a significant association using a multivariate regression model. However, the Easton et al. [89] model substantially overpredicted cancer risks in long-term workers (>10 years) who were employed between the years 1930-1939. This led the researchers to conclude that they may have "overestimated the risks for metallic (and possibly soluble) nickel and underestimated those for sulfides and/or oxides" [89]. A 2001 update of hydrometallurgical workers with relatively high metallic nickel exposures confirms the lack of excess respiratory cancer risk associated with exposures to elemental nickel during refining [90]. Review articles published in 2005 [91] and more recently in 2020 [92] have confirmed the earlier findings and not found associations between metallic nickel exposure and increased lung cancer risks.
Animal data on carcinogenicity are largely in agreement with the human data. Early studies on the inhalation of metallic nickel powder, although somewhat limited with respect to experimental design, are essentially negative for carcinogenicity [93, 94]. While intratracheal instillation of nickel metal powder has been shown to produce tumors in the lungs or mediastinum of animals [95, 96], the relevance of such studies in the etiology of lung cancer in humans is questionable. This is because normal defense systems and clearance mechanisms operative via inhalation are by-passed in intratracheal studies. Moreover, high mortality in one of the studies [96] suggests that toxicity could have confounded the carcinogenic finding in this study. Driscoll et al. [97] have cautioned that, in the case of intratracheal instillation studies, care must be taken to avoid doses that are excessive and may result in immediate toxic effects to the lung due to a large bolus delivery.
A definitive animal carcinogenicity study with inhalable nickel metal powder (~1.8 µm MMAD, 2.4 µm GSD) by inhalation in male and female Wistar rats was conducted using a 2-year regimen of exposure at 0, 0.1, 0.4, and 1 mg/m3. Toxicity and lethality required the termination of the 1 mg/m3. Nevertheless, the 0.4 mg/m3 group established the required Maximum Tolerated Dose (MTD) for inhalation of nickel metal powder and hence, was valid for the determination of carcinogenicity. This study, conducted according to OECD guidelines and GLP, did not show an association between nickel metal powder exposure and respiratory tumors [11], at workplace equivalent exposures up to 1.5-4.6 mg Ni/m3 inhalable Ni (Nickel CSR 2019, Appendix C2).
These data, in concert with the most recent epidemiological findings and a separate negative oral carcinogenicity study with a water-soluble nickel salt (most bioavailable form of nickel), strongly indicate that nickel metal powder is not likely to be a human carcinogen by any relevant route of exposure. Indeed, a recent systematic review of the epidemiological, animal and mechanistic evidence concluded that “the evidence does not support a causal relationship between metallic nickel exposure and respiratory cancer in humans” [92].
With respect to non-malignant respiratory disease, no convincing reports of asthma or fibrosis have been reported in workers with metallic nickel exposures. In the case of asthma, exposure to fine dust containing nickel has only infrequently been reported in anecdotal publications as a possible cause of occupational asthma [98-100]. Such dust exposures, however, have almost certainly included other confounding agents. Furthermore, no quantitative relationship has been readily established between the concentration of nickel cations in aqueous solution in bronchial challenge tests and equipotent metallic nickel in the occupational environment. In a U.S. study of welders (exposed to fumes containing complex spinels and other metals, with minute amounts of metallic nickel) at a nuclear facility in Oak Ridge, Tennessee, no increased mortality due to asthma was found among the workers studied [86]. Collectively, therefore, the overall data for metallic nickel being a respiratory sensitizer are not compelling, although a definitive study is lacking.
In addition to the unconvincing and very small number of anecdotal case-reports regarding asthma, a few other respiratory effects due to metallic nickel exposures have also been reported. Data relating to respiratory effects associated with short-term exposure to metallic nickel are very limited. One report exists of a fatality involving a man spraying nickel using a thermal arc process [12]. This man was exposed to very fine particles or fumes, likely consisting of metallic nickel or oxidic nickel. He died 13 days after exposure, having developed pneumonia, with postmortem showing shock lung. However, the relevance of this case to normal daily occupational exposures is questionable given the reported extremely high exposure (382 mg Ni/m3) to relatively fine nickel particles.
A few other studies have investigated the effects of nickel exposure on pulmonary function and fibrosis. With respect to pulmonary function, Kilburn et al. [101] examined cross-shift and chronic pulmonary effects in a group of stainless steel welders (with predominant nickel exposures to complex oxides but possibly some minute metallic nickel exposure). No differences in pulmonary function were observed in test subjects versus controls during cross-shift or short-term exposures. Although some reduced vital capacities were observed in long-term workers, the authors noted little evidence of chronic effects on pulmonary function caused by nickel. Conversely, in studies of stainless steel and mild steel welders, short-term, cross-shift effects were noted in stainless steel workers (reduced FEV1:FVC[1] ratio), but no long-term effects in lung function were noted in workers with up to 20 years of welding activity [102, 103]. A generalized decrease in lung function, however, was seen in workers with the longest histories (over 25 years) of stainless steel welding. This was attributed to the high concentrations of mixed pollutants (i.e., dust, metal oxides, and gasses) to which these welders were exposed. A higher prevalence of bronchial irritative symptoms, such as cough, was also reported.
With respect to fibrosis, a study on nickel refinery workers in Norway examined the incidence of X-ray abnormalities (ILO ³ 1/0) [20]. The incidence of irregular opacities in X-rays was not significantly different from the hospital incidence in “normal” X-rays (4.5% vs 4.2%, respectively). An increased risk of abnormal X-rays was found with cumulative exposure to sulfidic and soluble, but not for oxidic or metallic nickel [20].
Animal studies on the non-carcinogenic respiratory effects of metallic nickel are few. The early studies by Heuper and Payne [94] suggest that inflammatory changes in the lung can be observed in rats and hamsters administered nickel powder via inhalation. However, lack of details within the studies precluded drawing any conclusions with respect to the significance of the findings. In the 2-year cancer bioassay study [11], chronic inflammation was observed in rats exposed to nickel metal powder at ≥ 0.1 mg/m3 (MMAD 1.8 µm, GSD 2.4). Studies on the effects of ultrafine metallic nickel powder (mean diameter of 20 nm) administered intratracheally or via short-term inhalation in rats showed significant inflammation, cytotoxicity, and/or increased epithelial permeability of lung tissue [104, 105].
Collectively, the above findings present a mixed picture with respect to the potential risk of non-malignant respiratory disease from metallic nickel exposures. There is an extensive body of literature demonstrating that past exposures to metallic nickel have not resulted in excess mortality from such diseases [14, 15, 85-88, 90, 106]. Studies of welders may be less relevant for metallic nickel, as exposures are predominantly to complex Ni-metal oxides (spinels), rather than nickel metal. However, additional studies on such effects, particularly with respect to ultrafine nickel powders, would be useful.
4.1.2 Dermal Exposure: Metallic Nickel
Dermal exposure to metallic nickel is possible wherever nickel powders are handled, such as powder metallurgy, and in the production of nickel-containing batteries, chemicals, and catalysts. Occasional contact with massive forms of metallic nickel could occur during nickel metal production, alloy production, production of articles made of nickel metal or alloys, and use of nickel-containing articles.
Skin sensitization to nickel metal can occur wherever there is sufficient leaching of nickel ions from articles containing nickel onto exposed skin [107, 108]. However, cutaneous allergy (allergic contact dermatitis) to nickel occurs mainly as the result of non-occupational exposures. Indeed, the evidence for occupationally-associated nickel allergic reactions is sparse [52, 109-111] due in large part to increased occupational hygiene measures.
Sensitization and subsequent allergic reactions to nickel require direct and prolonged contact with nickel-containing solutions or nickel-releasing items that are non-resistant to sweat corrosion (see further discussion under Sections 5.2 and 5.4). The nickel ion must be released from a nickel-containing article in intimate contact with skin to elicit a response. Evidence suggests that humid environments are more likely to favor the release of the nickel ion from metallic nickel and nickel alloys, whereas dry, clean operations with moderate or even intense contact to nickel objects will seldom, alone, provoke dermatitis [52]. In some occupations for which nickel dermatitis has been reported in higher proportion than the general populace (e.g., cleaning, hairdressing and hospital wet work), the wet work is, in and of itself, irritating and decreases the barrier function of the skin. Often it is the combination of irritant dermatitis and compromised skin barrier that produces the allergic reaction [52]. The role of nickel in the manifestation of irritant dermatitis in metal manufacturing, cement and construction industries, and coin handling has been debated. It has been suggested by some researchers that nickel probably does not elicit dermatitis in workers from such industries unless the worker is already strongly allergic to nickel [52]. There are some reports that oral ingestion of high nickel levels (above 12 µg/kg/day) can trigger a dermatitis response in susceptible nickel-sensitized individuals (see section 5.3.3).
4.2 NICKEL ALLOYS
Often there is a misconception that the toxicity of nickel-containing alloys is synonymous with the toxicity of metallic nickel. This is not necessarily true. Each type of nickel-containing alloy is a unique substance with its own special physico-chemical and biological properties that differ from those of its individual metal constituents. Alloy constituents can affect the release of nickel metal, increasing or decreasing it from what would be expected based on nickel metal content, changing the toxicity profile of the alloy. The potential toxicity of a nickel alloy (including carcinogenic effects) must, therefore, be evaluated separately from the potential toxicity of nickel metal itself and other nickel-containing alloys.
While there are hundreds of different nickel-containing alloys in different product categories, the major product categories are stainless steel (containing Fe, Cr and up to 34% Ni) and high nickel content alloys. Occupational exposures to nickel from these and other forms of nickel alloys (e.g., superalloys, cast-irons) can occur wherever alloys are produced (metallurgical operations) or in the processing of alloys (such as welding, grinding, cutting, polishing, and forming). Like metallic nickel, occupational exposures to nickel-containing alloys will mainly be via the skin or through inhalation. However, in the case of certain nickel alloys that are used in prosthetic devices, localized internal exposures can occur. Because such exposures are not of specific concern to occupational settings, they are not discussed in this Guide. However, a comprehensive review of information pertaining to prosthetic devices can be found in e.g., [112, 113].
4.2.1 Inhalation Exposure: Nickel Alloys
There are no studies of nickel workers exposed solely to nickel alloys in the absence of metallic or oxidic nickel. Clearly, however, workers in alloy and stainless steel manufacturing and processing will likely have some low level exposure to nickel alloys. In general, most studies on stainless steel and nickel alloy workers have shown no significant occupationally-related excess risks of respiratory cancer [14, 15, 85, 86, 114-118]). As noted above and in the discussion on metallic nickel, some of these studies involved thousands of workers [14]. Hence, these studies suggest an absence of nickel-related excess cancer risks in workers exposed to nickel-containing alloys.
There have been some exceptions, however, in certain groups of stainless steel welders [119, 120] where excess lung tumors were detected. Further analyses of these and other stainless steel workers as part of a large international study on welders (> 11,000 workers) failed to show any association between increased lung cancer mortality and cumulative exposure to nickel [121]. A later analysis of this same cohort [122] showed no trend for lung cancer risk for three levels of nickel exposure. Likewise, no nickel-related tumors were observed in a group of German arc welders exposed to fumes containing chromium and nickel [123]. In 2017, IARC reviewed the evidence for the carcinogenicity of welding fumes and its components and concluded that welding fumes as a whole are Group 1 carcinogens, but did not distinguish between stainless or mild steel welding [124]. Importantly, the exposures during welding are mainly to complex oxides (spinels) of very small particle size with minor contributions from nickel alloys or metal.
Limited data are available to evaluate respiratory carcinogenicity of nickel alloys in animals. One intratracheal instillation study looked at two types of stainless steel grinding dust. An austenitic stainless steel (6.8% nickel) and a chromium ferritic steel (0.5% nickel) were negative in hamsters after repeated instillations [125]. In another study, grinding dust from an austenitic stainless steel (26.8% nickel) instilled in hamsters was also negative [96]. In this same study, an alloy containing 66.5% nickel, 12.8% chromium, and 6.5% iron showed some evidence of carcinogenic potential at the higher doses tested. A significant shortening in survival time in one of the high dose groups compared to untreated controls, however, raises the question of toxicity and its possible confounding effect on tumor formation. As noted in the discussion of metallic nickel, intratracheal instillation studies must be carefully interpreted in light of their artificial delivery of unusually large and potentially toxic doses of chemical agents to the lung [97].
In total, there is little evidence to suggest that nickel alloys, as such, act as respiratory carcinogens. For many alloys, this may be due to their corrosion resistance which results in reduced release of metal ions to target tissues.
With respect to non-carcinogenic respiratory effects, no animal data are available for determining such effects, and the human studies that have looked at such endpoints have generally shown no increased mortality due to non-malignant respiratory disease [14, 15, 85, 86, 114, 121].
4.2.2 Dermal Exposure: Nickel Alloys
Because alloys are specifically formulated to meet the need for manufactured products that are durable and corrosion resistant, an important property of all alloys and metals is that they are insoluble in aqueous solutions. They can, however, react (corrode) in the presence of other media, such as air or biological fluids, to form new metal-containing species that may or may not be water soluble. The extent to which alloys react is governed by their corrosion resistance in a particular medium and this resistance is dependent on the nature of the metals, the proportion of the metals present in the alloy, and the process by which the alloy was made.
Of particular importance to dermal exposures are the potential of individual alloys to corrode in sweat. As noted under the discussion of metallic nickel, sensitization and subsequent allergic reactions to nickel require direct and prolonged contact with nickel-containing solutions or materials that are non-resistant to sweat corrosion. It is the release of the nickel (II) ion, not the nickel content of an alloy, that will determine whether a response is elicited. Occupational dermal exposures to nickel alloys are possible wherever nickel alloy powders are handled, such as in powder metallurgy or catalyst production. While exposures to massive forms of nickel alloys are also possible in occupational settings, these exposures do not tend to be prolonged, and, hence, are not of greatest concern with respect to contact dermatitis. Dermal contact with nickel-copper alloys in coinage production can also occur. The potential for nickel alloys to elicit an allergic reaction in occupational settings, therefore, will depend on both the sweat resistant properties of the alloy and the amount of time that a worker is in direct and prolonged contact with an alloy.
While the EU Nickel Directive (94/27/EC), limiting the Ni release from alloys that come into close contact with the skin, is geared toward protecting the general public from exposures to nickel contained in consumer items, it may also provide some guidance in occupational settings where exposures to nickel alloys are direct and prolonged. It should be noted, however, that alloys that release greater than 0.5 ug/cm2/week of nickel may not be harmful in an occupational or commercial setting. They may be used safely when not in direct and prolonged contact with the skin or where ample protective clothing is provided. A comprehensive review of the health effects associated with the manufacture, processing, and use of stainless steel can be found in Cross et al. [126].
4.3 SOLUBLE NICKEL
Exposure to readily water-soluble nickel salts occurs mainly during the electrolytic refining of nickel (producing industries) and in electroplating (using industries). Depending upon the processes used, exposures are usually to hydrated nickel (II) sulfate or nickel chloride in solution. Like the previously mentioned nickel species, the routes of exposure of toxicological relevance to the workplace are inhalation and dermal exposures. However, unlike other nickel species, soluble nickel (II) ions are present in drinking water; thus, oral exposures are briefly mentioned below.
4.3.1 Inhalation Exposure: Soluble Nickel
Like metallic nickel, the two effects of greatest concern for the inhalation of soluble nickel compounds are respiratory cancer and non-malignant respiratory effects (e.g., fibrosis, asthma). Unlike metallic nickel, however, which has consistently shown lack of evidence of carcinogenicity, the carcinogenic assessment of soluble nickel compounds has been somewhat challenging. The challenge lies both in reconciling what appears to be inconsistent human data and in interpreting the human and animal data in an integrated manner that provides a cohesive picture of the carcinogenicity of soluble nickel compounds.
Human evidence for the carcinogenicity of soluble nickel compounds comes mainly from studies of nickel refinery workers in Wales, Norway, and Finland [24, 89, 127-129]. In these studies, workers involved in electrolysis, electrowinning, and hydrometallurgy have shown excess risks of lung and/or nasal cancer. Exposures to soluble nickel have generally been regarded to be relatively high in most of these workers (in excess of 1 mg Ni/m3), although some studies have suggested that exposures slightly lower than 1 mg Ni/m3 may have contributed to some of the cancers observed [128, 130]. In all instances, soluble nickel exposures in these workers have been confounded by concomitant exposures to other nickel compounds (notably, oxidic and sulfidic nickel compounds), other chemical agents (e.g., soluble cobalt compounds, arsenic, acid mists) or cigarette smoking-all known or believed to be potential carcinogens in and of themselves (see Sections 5.4 and 5.5). Therefore, it is unclear whether soluble nickel, alone, caused the excess cancer risks seen in these workers.
In contrast to these workers, electrolysis workers in Canada and plating workers in the U.K. have shown no increased risks of lung cancer [24, 131-133]. In the case of the Canadian electrolysis workers, their soluble nickel exposures were similar to those of the electrolysis workers in Norway. Soluble nickel exposures in the plating workers, although unknown, are presumed to have been lower. On the whole, these workers were believed to lack, or have lower exposures to, some of the confounding agents present in the work environments of the workers mentioned above. While nasal cancers were seen in a few of the Canadian electrolysis workers, these particular workers had also worked in sintering departments where exposures to sulfidic and oxidic nickel were very high (> 10 mg Ni/m3). It is likely that exposures to the latter forms of nickel (albeit some of them short) may have contributed to the nasal cancers observed (see Sections 5.4 and 5.5).
Besides the epidemiological studies, the animal data also needs to be considered. The most important inhalation animal studies conducted to date are those of the U.S. National Toxicology Program. In these studies, nickel subsulfide, nickel sulfate hexahydrate, and a high-temperature nickel oxide were administered to rats and mice in two-year carcinogenicity bioassays [19, 21, 134]. Results from the nickel sulfate hexahydrate study [19] are particularly pertinent to the assessment of the carcinogenicity of soluble nickel compounds. This 2-year chronic inhalation study failed to produce any carcinogenic effects in either rats or mice at exposures to nickel sulfate hexahydrate up to 0.11 mg Ni/m3 or 0.22 mg Ni/m3, respectively [19]. These concentrations correspond to approximately 0.70-2.0 mg Ni/m3 workplace aerosols after adjusting for particle size and animal to human extrapolation [43, 135, 136]. It is also worth noting that soluble nickel compounds administered via other relevant routes of exposure (oral) in lifetime carcinogenicity studies have also failed to produce tumors [65, 137-139].
In sum, the negative animal data combined with the conflicting human data make for an uncertain picture regarding the carcinogenicity of soluble nickel alone.
As noted by Oller [140], without a unifying mode of action that can both account for the discrepancies seen in the human data and integrate the results from human and animal data into a single model for nickel respiratory carcinogenesis, assessments of soluble nickel will continue to vary widely. Such a MoA has been proposed in models for nickel-mediated induction of respiratory tumors. These models suggest that the main determinant of the respiratory carcinogenicity of a nickel species is likely to be the bioavailability of the nickel (II) ion at nuclear sites of target epithelial cells [141-144]. Only those nickel compounds that result in sufficient amounts of bioavailable nickel (II) ions at such sites (after inhalation) will be respiratory carcinogens. Because soluble nickel compounds are not phagocytized and are rapidly cleared, substantial amounts of nickel (II) ions that would cause tumor induction simply are not present.
However, at workplace equivalent levels above 0.19-0.26 mg Ni/m3 [43] chronic respiratory toxicity was observed in animal studies [19]. Respiratory toxicity due to soluble nickel exposures may have enhanced the induction of tumors by less soluble nickel compounds or other inhalation carcinogens seen in refinery workers. This may account for the observed respiratory cancers seen in the Norwegian, Finnish, and Welsh refinery workers who had concomitant exposures to smoking and other inhalation carcinogens. Indeed, in its multi-analysis of many of the nickel cohorts discussed above, the International Committee on Nickel Carcinogenesis in Man (ICNCM) postulated that the effects of soluble nickel may be to enhance the carcinogenic process, as opposed to inducing it [24]. Alternatively, it should be considered that none of the workers in the sulfidic ore refinery studies had pure exposures to soluble nickel compounds that did not include sulfidic or complex nickel oxides, and most of them had confounding by smoking and in some cases arsenic or cobalt.
To identify a practical lung cancer threshold for exposure to the main chemical forms of nickel, the dose-response (D-R) for soluble and oxidic compounds were analyzed by Oller et al. [18], taking into account differences in response relative to the presence of sulfidic and oxidic Ni exposure levels above and below 0.2 mg Ni/m3 (as inhalable aerosol fraction). The (measured or estimated) exposures (corrected to inhalable) and risk ratios from Goodman et al. [142] were used. In total, lung cancer data from 22 process areas arising from 13 cohorts of geographically distinct nickel producing and using operations were included, encompassing >100,000 workers. Based on these data, a practical threshold of inhalable aerosol fraction of 0.10 mg Ni/m3 soluble Ni (with ≤ 0.2 mg Ni/m3 of oxidic and sulfidic Ni) can be conservatively applied to all forms of nickel.
Animal inhalation studies have shown various non-malignant respiratory effects on the lung following relatively short periods of exposure to relatively high levels of soluble nickel compounds [44, 75, 145-148]). Effects have included marked hyperplasia, inflammation and degeneration of bronchial epithelium, increased mucus secretion, and other indicators of toxic damage to lung tissue. In a study where nickel sulfate was administered via a single intratracheal instillation in rats, the nickel sulfate was shown to transiently affect pulmonary antitumoral immune defenses [149]. Chronic exposures to nickel sulfate hexahydrate result in cell toxicity and inflammation [19]. Moreover, a subchronic study demonstrated that nickel sulfate hexahydrate has a steep dose-response for toxicity and mortality [150]. Hence, although exposure to soluble nickel compounds, alone, may not provide the conditions necessary to cause cancer (i.e., the nickel (II) ion is not delivered to the target tissue in sufficient quantities in vivo), due to their toxicity, soluble nickel compounds may enhance the carcinogenic effect of other nickel compounds or cancer-causing agents by increasing cell proliferation. Cell proliferation, in turn, is required to convert DNA lesions into mutations and expand the mutated cell population, resulting in carcinogenesis.
With respect to non-malignant respiratory effects in humans, the evidence for soluble nickel salts being a causative factor for occupational asthma, while not overwhelming, is more suggestive than it is for other chemical forms of nickel. Such evidence arises mainly from a small number of case reports in the electroplating industry and nickel catalyst manufacturing [151-156]. Exposure to nickel sulfate can only be inferred in some of the cases where exposures have not been explicitly stated. Many of the plating solutions and, hence, aerosols to which some of the workers were exposed may have had a low pH. This latter factor may contribute to irritant effects which are not necessarily specific to nickel. In addition, potential for exposure to other sensitizing metals, notably chromium and cobalt, may have occurred. On the basis of the studies reported, the frequency of occupational asthma cannot be assessed, let alone the dose response determined. Despite these shortcomings, however, the role of soluble nickel as a possible cause of asthma should be considered.
Aside from asthma, the only other non-carcinogenic respiratory effect reported in nickel workers is that of fibrosis. Evidence that soluble nickel may act to induce pulmonary fibrosis at the radiological level comes from a study of nickel refinery workers that showed modest abnormalities in the chest x-rays of workers [20]. Berge and Skyberg identified a dose-response trend for 4 categories of cumulative exposure to soluble Ni. However, there was also evidence that other factors (e.g., age and tobacco consumption) were more reliable predictors of the cohort’s incidence of radiographically-identified fibrosis. Thus, the odds ratio for the group with the highest cumulative exposure to soluble Ni lost statistical significance when it was adjusted for age, smoking, asbestos and sulphidic Ni exposure (OR = 2.24, 95% CI 0.82-6.16). The significance of these results for the clinical diagnosis of fibrosis remains to be determined as X-ray findings have been reported to not correlate well with functional diagnosis of lung fibrosis [157].
4.3.2 Dermal Exposure: Soluble Nickel
Historically, risks for allergic contact nickel dermatitis have been elevated in workplaces where exposures to soluble nickel have been high. For example, nickel dermatitis was common in the past among nickel platers. However, due to improved industrial and personal hygiene practices, more recent reports of nickel sensitivity in workplaces such as the electroplating industry have been sparse. Schubert et al., [158] found only two nickel sensitive platers among 176 nickel sensitive individuals studied. A number of studies have shown nickel sulfate to be a skin sensitizer in animals, particularly in guinea pigs [159-162]. Dermal studies in animals suggest that sensitization to soluble nickel (nickel sulfate) may result in cross sensitization to cobalt [163] and that oral supplementation with zinc may lessen the sensitivity reaction of NiSO4-induced allergic dermatitis [164]. Soluble nickel compounds should be considered skin sensitizers in humans and care should be taken to avoid prolonged contact with nickel solutions in the workplace.
Allergic contact dermatitis is the most prevalent effect of nickel in the general population. Epidemiological investigations have shown that prevalence of nickel allergy is approximately 14.5% of the general population in several European countries [165]. Significantly decreased prevalence of nickel allergy has been observed in the younger European population, born since the institution of regulation of nickel release from consumer articles used for piercing and intended for direct and prolonged skin contact in the late 1990s (the EU nickel directive), with this Directive being included in the European REACH regulation as entry 27 in Annex XVII in 2009 [166].
4.3.3 Other Exposures: Soluble Nickel
The evidence for the lack of oral carcinogenicity of nickel substances is conclusive. In a study by Heim et al. [137], nickel sulfate hexahydrate was administered daily to rats by oral gavage for 2 years (104 weeks) at exposure levels of 10, 30 and 50 mg NiSO4•6H2O/kg. This treatment produced a statistically significant reduction in body weight of male and female rats, compared to controls, in an exposure-related fashion at 30 and 50 mg/kg/day. An exposure-dependent increase in mortality was observed in female rats. However, daily oral administration of nickel sulfate hexahydrate did not produce an exposure-related increase in any common tumor type or an increase in any rare tumors. This study achieved sufficient toxicity to reach the Maximum Tolerated Dose (MTD) while maintaining a sufficiently high survival rate to allow evaluation for carcinogenicity. The study by Heim et al. [137] demonstrates that nickel sulfate hexahydrate does not have the potential to cause carcinogenicity by the oral route of exposure. Data from this and other studies demonstrate that inhalation is the only route of exposure that may cause concern for cancer in association with nickel compound exposures.
Unlike other species of nickel, oral exposure to soluble nickel (II) ions occurs from drinking water (and from bioavailable nickel present in food). Data from both human and animal studies show that absorption of nickel from food and water is generally low (1-30%), depending on the fasting state of the subject, with most of the nickel excreted in feces [167]. In humans, effects of greatest concern for ingested nickel are those produced in the kidney, possible reproductive effects, and the potential for soluble nickel to exacerbate nickel dermatitis following oral provocation.
Several researchers have examined the evidence of nephrotoxicity related to long-term exposures to soluble nickel in electroplating, electrorefining and chemical workers [168-171]. These workers not only would have been exposed to soluble nickel in their food and water, but also in the workplace air which they breathed. Wall and Calnan [170] found no evidence of renal dysfunction among 17 workers in an electroplating plant. Likewise, Sanford and Nieboer [169], in a study of 26 workers in electrolytic refining plants, concluded that nickel, at best, might be classified as a mild nephrotoxin. In the Sunderman and Horak study [168] and the Vyskočil et al., study [171], elevated markers of renal toxicity (e.g., β2 microglobulin) were observed, but only spot urinary nickel samples were taken. The chronic significance of these effects is uncertain. In addition, nickel exposures were quite high in these workers (up to 13 mg Ni/m3 in one instance), and certainly not typical of most current occupational exposures to soluble nickel. Severe proteinuria and other markers of significant renal disease that have been associated with other nephrotoxicants (e.g., cadmium) have not been reported in nickel workers, despite years of biological monitoring and observation. However, a 2020 case-control study suggested an association, albeit tenuous, between chronic, low dose environmental exposure to nickel and acute Mesoamerican Nephropathy [172]. In animals, kidney toxicity was observed 28 days after gavage treatment of mice with 30 mg/kg nickel chloride [173] and in rats, kidney damage was observed 20 days after intraperitoneal injection of 20 mg/kg bw/day nickel [174].
In regard to reproductive effects, there is some evidence in humans to indicate that absorbed nickel may be able to move across the placenta into fetal tissue [175-177]. An early study of Russian nickel refinery workers purported to show evidence of spontaneous abortions, stillbirths, and structural malformations in babies born to female workers at that refinery [178]. Concerns about the reliability of this study prompted a more thorough and well-conducted epidemiology investigation of the reproductive health of the Russian cohort that was also important for another reason. Specifically, the nickel refineries in this region are the only places worldwide where enough female nickel refinery workers exist to perform an epidemiological survey of reproductive performance at relatively high nickel exposures. In order to accomplish this task, the researchers constructed a birth registry for all births occurring in the region during the period of the study. They also reconstructed an exposure matrix for the workers at the refinery so as to be able to link specific pregnancy outcomes with occupational exposures. The study culminated in a series of manuscripts by A. Vaktskjold et al. [179-184] describing the results of the investigation. The study demonstrated that nickel exposure was not correlated with adverse pregnancy outcome for 1) male newborns with genital malformations, 2) spontaneous abortions, 3) small-for-gestational-age newborns, or 4) musculosketal effects in newborns of female refinery workers exposed to nickel. The lack of a “small-for-gestational-age” and “male genital malformation” findings are considered “sentinel” effects (i.e., sensitive endpoints) for reproductive toxicity in humans. These manuscripts showed no correlation between nickel exposure and observed reproductive impairment. These are important results as spontaneous abortion in humans would most closely approximate the observation of perinatal lethality associated with nickel exposure in rodents.
While the work by Vaktskjold et al. [181-184] is important in demonstrating that any risk of reproductive impairment from nickel exposure is exceedingly small, it should be noted that it is not possible to find women whose occupational nickel exposure persisted throughout their pregnancies until birth. Generally, fetal protection policies require removal of pregnant women from jobs with exposures to possible reproductive toxicants. Therefore, it cannot be concluded that occupational exposure to nickel compounds during pregnancy present no risk, only that any risk is exceedingly small.
With respect to animal studies, a variety of developmental, reproductive, and teratogenic effects have been reported in animals exposed mainly to soluble nickel via oral and parenteral administration [177]. However, factors such as high doses, relevance of routes of exposure, avoidance of food and water, lack of statistical significance, and parental mortality have confounded the interpretation of many of the results [177, 185]. No malformations (i.e., teratogenesis) were identified in a rat prenatal developmental toxicity study with nickel chloride at the maximum tolerated dose of 42 mg Ni/kg bw/day [186, 187], but nickel chloride was shown to cause malformations (e.g., microphthalmia) in a prenatal developmental toxicity study in mice at 46 mg/kg bw/day and other teratogenic effects were evident at higher doses [188].
In the most recent and reliable reproductive study conducted to date, rats were exposed to various concentrations of nickel sulfate hexahydrate by gavage [189, 190]. In the 1-generation range finding study, evaluation of post-implantation/perinatal lethality among the offspring of the treated parental rats (i.e., number of pups conceived minus the number of live pups at birth) showed statistically significant increases at the 6.6 mg Ni/kg/day exposure level and questionable increases at the 2.2 and 4.4 mg Ni/kg/day levels. The definitive 2-generation study demonstrated that these effects were not evident at concentrations up to 1.1 mg Ni/kg/day soluble nickel. Based on these studies a BMDL10 or BMDL5 of 1.3 or 1.8 mg Ni/kg/day were calculated by EFSA [7] and Haber et al [191], respectively. No nickel effects on fertility, sperm quality, estrous cycle and sexual maturation were found in these studies [189, 190].
Nickel dermatitis via oral exposure only occurs in individuals already sensitized to nickel via dermal contact, and in only a very small portion of nickel-sensitized individuals. Studies suggest that only a minor number of nickel sensitive patients react to oral doses below 1.25 mg of nickel (~20 µg Ni/kg). These doses are in addition to the normal dietary nickel intake (~160 µg Ni/day). Systemically induced flares of dermatitis have been reported after oral challenge of nickel-sensitive women with 0.5-5.6 mg of nickel as nickel sulfate administered in a lactose capsule [192]. At the highest nickel dose (5.6 mg), there was a positive reaction in majority of the subjects; at 0.5 mg, only a few persons responded with flares. Responses to oral doses of 0.4 or 2.5 mg of nickel did not exceed responses in subjects given placebos in double-blind studies [193, 194]. The Lowest Observed Adverse Effects Level (LOAEL) for exacerbation of nickel dermatitis symptoms in nickel-sensitized individuals established by EFSA in their Update of the risk assessment of nickel in food and drinking water [7] was 4.3 μg Ni/kg body weight (assuming a body weight of 70 kg), based on the study by Jensen et al. [195]. For nickel-sensitized individuals who are susceptible to orally-induced nickel dermatitis, a low nickel diet or oral hyposensitization have been investigated. Various low nickel diets have been developed, providing lists of foods to avoid and to eat based on nickel content [196, 197]. Oral hyposensitization to nickel using nickel sulphate has also been demonstrated to improve dermatitis symptoms in nickel-sensitized individuals in multiple studies [198-201].
Conversely, oral exposure to nickel in non-nickel-sensitized individuals has been shown to provide tolerance to future dermal nickel sensitization. Observations first made in animal experiments [202] and correlations obtained from studies of human cohorts [203] led to the hypothesis that nickel hypersensitivity reactions may be prevented by prior oral exposure to nickel if long-term, low-level antigenic contact occurs in the non-sensitized organism. Studies that followed van der Burg's initial observation of induced nickel tolerance in humans have repeatedly confirmed the occurrence of this phenomenon both in humans [204-208] and animals [209, 210]. Suppression of dermal nickel allergic reactions can also be achieved in sensitized individuals [201].
4.4 OXIDIC NICKEL
The term “oxidic nickel” includes nickel (II) oxides, nickel (III) oxides, possibly nickel (IV) oxides and other non-stochiometric entities, complex nickel oxides (including spinels in which other metals such as copper, chromium, or iron are present), silicate oxides (garnierite), hydrated oxides, hydroxides, and, possibly, carbonates or basic carbonates which are subject to various degrees of hydration. Therefore, for the purposes of this document they will be considered together.
Oxidic nickel is used in many industrial applications and will be present in virtually every major nickel industry sector. Nickel oxide sinter is often the end product in the roasting of nickel sulfide concentrates. It is used as charge to produce wrought stainless steel and other alloy materials. It is also used in cast stainless steel and nickel-based alloys. Commercially available nickel oxide powders are used in the electroplating industry, for catalysis preparation, and for other chemical applications. Black nickel oxide and hydroxide are used in the production of electrodes for nickel-cadmium batteries utilized in domestic markets and also in large power units. Complex nickel oxides are used in oil refining and ceramic magnets [211, 212].
Like the previously discussed nickel species, inhalation of oxidic nickel compounds is the route of exposure of greatest toxicological concern in occupational settings. Unlike the former species of nickel, however, dermal exposures to oxidic nickel are believed to be of little consequence to nickel workers. While no data are directly available on the effects of oxidic nickel compounds on skin, due to their low water solubility, very low absorption of nickel through the skin is expected.
4.4.1 Inhalation Exposure: Oxidic Nickel
The critical health effect of interest in relation to occupational exposure to oxidic nickel is, again, respiratory cancer. Unlike metallic nickel, which does not appear to be carcinogenic, there is evidence for the carcinogenicity of certain oxidic nickel compounds even though there is still some uncertainty regarding the forms of oxidic nickel that induce tumorigenic effects. Although oxidic nickel is present in most major industry sectors, it is of interest to note that epidemiological studies have not consistently implicated all sectors as being associated with respiratory cancer. Indeed, excess respiratory cancers have been observed only in refining operations in which nickel oxides were produced during the refining of sulfidic ores and where exposures to oxidic nickel were relatively high (> 5 mg Ni/m3) [24]. At various stages in this process, nickel-copper oxides may have been formed. In contrast, no excess respiratory cancer risks have been observed in workers exposed to lower levels (< 2 Ni/m3) of oxidic nickel free of copper during the refining of lateritic ores or in the nickel-using industry.
Specific operations where oxidic nickel was present and showed evidence of excess respiratory cancer risk include refineries in Kristiansand, Norway, Clydach, Wales, and Copper Cliff and Port Colborne, Ontario, Canada. In all instances, workers were exposed to various combinations of sulfidic, oxidic, and soluble nickel compounds. Nevertheless, conclusions regarding the carcinogenic potential of oxidic nickel compounds have been gleaned by examining those workers predominantly exposed to oxidic nickel.
In the case of Kristiansand, this has been done by examining workers in the roasting, smelting and calcining department [24] and by examining all workers by cumulative exposure to oxidic nickel [24, 127]. In the overall cohort, there was evidence to suggest that long-term exposure (³15 years) to oxidic nickel (mainly nickel-copper oxides at concentrations of 5 mg Ni/m3 or higher) was related to an excess of lung cancer. There was also some evidence that exposure to soluble nickel played a role in increasing cancer risks in these workers (see Section 5.3). The effect of cigarette smoking has also been examined in these workers [127, 213], with the Grimsrud [213] study showing a multiplicative effect (i.e., interaction) between cigarette smoking and exposure to nickel. Evidence of excess nasal cancers in this group of workers has been confined to those employed prior to 1955. This evidence suggests that oxidic nickel has been a stronger hazard for nasal cancer than soluble nickel, as 12 cases (0.27 expected) out of 32 occurred among workers exposed mostly to nickel oxides.
In the Welsh and Canadian refineries, workers exposed to some of the highest levels (10 mg Ni/m3 or higher) of oxidic nickel included those working in the linear calciners and copper and nickel plants (Wales) and those involved in sintering operations in Canada. In Wales, oxidic nickel exposures were mainly to nickel-copper oxides or impure nickel oxide; in Canada, exposures were mainly to high-temperature nickel oxide with lesser exposure to nickel-copper oxides. Unfortunately, in the latter case, oxidic exposures were completely confounded by sulfidic nickel exposures, making it difficult to distinguish between the effects caused by these two species of nickel. Both excess lung and nasal cancer risks were seen in the Welsh and Canadian workers [24, 129, 132].
In contrast to the above refinery studies, studies of workers mining and smelting lateritic ores (where oxidic nickel exposures would have been primarily to silicate oxides and complex nickel oxides free of copper) have shown no evidence of nickel-related respiratory cancer risks. Studies by Goldberg et al. [214, 215] of smelter workers in New Caledonia showed no evidence of increased risk of lung or nasal cancer at estimated exposures of 2 mg Ni/m3 or less. Likewise, in another study of smelter workers in Oregon, there was no evidence of excess nasal cancers [24]. While there were excess lung cancers, these occurred only in short-term workers, not long-term workers. Hence, there was no evidence to suggest that the lung cancers observed were related to the low concentrations (£ 1 mg Ni/m3) of oxidic nickel to which the men were exposed [24].
In nickel-using industries, the evidence for respiratory cancers has also largely been negative. As noted in previous sections (Sections 5.1 and 5.2), most studies on stainless steel and nickel alloy workers that would have experienced some level of exposure to oxidic nickel have shown no significant nickel-related excess risks of respiratory cancer [14, 15, 85, 86, 114-118, 121, 122]. In Swedish nickel-cadmium battery workers, there is some evidence of an increased incidence of nasal cancers, but it is not clear whether this is due to exposure to nickel hydroxide, cadmium oxide, or a combination of both [216]. In addition, little is known about the previous employment histories of these workers. It is, therefore, not clear whether past exposures to other potential nasal carcinogens may have contributed to the nasal cancers observed in these workers. In contrast, no nickel-related increased risk for lung cancer has been found in these or other nickel-cadmium battery workers [216-220].
From the overall epidemiological evidence, it is possible to speculate that the composition of oxidic nickel associated with an increase of lung or nasal cancer may primarily be nickel-copper oxides produced during the roasting and electrorefining of sulfidic nickel-copper mattes. However, careful scrutiny of the human data also reveals that high respiratory cancer risks occurred in sintering operations-where exposures to nickel-copper oxides would have been relatively low-and, possibly, in nickel-cadmium battery workers, where oxidic exposures would predominantly have been to nickel hydroxide. In addition to the type of oxidic nickel, the level to which nickel workers were exposed must also be taken into consideration. Concentrations of oxidic nickel in the high-risk cohorts (those in Wales, Norway, and Port Colborne and Copper Cliff, Canada) were considerably higher than those found in New Caledonia, Oregon, and most nickel-using industries. In the case of the nickel-cadmium battery workers, the early exposures that would have been critical to the induction of nasal cancers of long latency were believed to have been relatively high (> 2 mg Ni/m3). Hence, it may be that there are two variables—the physicochemical nature of the oxide and the exposure level—that contribute to the differences seen among the various cohorts studied.
Animal data shed some light on the matter. In the previously mentioned NTP studies, nickel oxide was administered to rats and mice in a two-year carcinogenicity bioassay [21]. The nickel oxide used was a green, high-temperature nickel oxide calcined at 1,350°C; it was administered to both rats and mice for 6 hours/day, 5 days/week for 2 years. Rats were exposed to concentrations of 0, 0.5, 1.0, or 2.0 mg Ni/m3. These concentrations are equivalent to 0.2 to 3.2 mg Ni/m3 inhalable workplace aerosol after adjusting for particle size differences and animal to human extrapolation [43, 135, 136]. After two years, no increased incidence of tumors was observed at the lowest exposure level in rats (equivalent to 0.23-0.81 mg Ni/m3 inhalable). At the intermediate and high concentrations, 12 out of 106 rats and 9 out of 106 rats, respectively, presented with either adenomas or carcinomas. On the basis of these results, the NTP concluded that there was some evidence of carcinogenic activity in rats. In contrast, there was no evidence of treatment-related tumors in male mice at any of the doses administered (1.0, 2.0 and 4.0 mg Ni/m3) and only equivocal evidence in female mice exposed to 1.0 but not 2.0 or 4.0 mg Ni/m3.
Carcinogenic evidence for other oxidic nickel compounds comes from animal studies using routes of exposure that are not necessarily relevant to man (i.e. intratracheal instillation, injection). In these studies, nickel-copper oxides appear to be as potent as nickel subsulfide in inducing tumors at injection sites [22]. There is, however, no strong evidence to indicate that black (low temperature) and green (high temperature) nickel oxides differ substantially with regard to tumor-producing potency. Some forms of both green and black nickel oxide produce carcinogenic responses, while other forms have tested negative in injection and intratracheal studies [22, 95, 221-226].
On the whole, comparisons between human and animal data suggest that certain oxidic nickel compounds at high concentrations may increase respiratory cancer risks and that these risks are not necessarily confined to nickel-copper oxides. However, there is no single unifying physical characteristic that differentiates oxidic nickel compounds with respect to biological reactivity or carcinogenic potential. Some general physical characteristics which may be related to carcinogenicity include: particle size £ 5 µm, a relatively large particle surface area, presence of metallic or other impurities and/or amount of Ni (II). Phagocytosis appears to be a necessary, but not sufficient condition for carcinogenesis. Solubility in biological fluids will also affect how much nickel ion is delivered to target sites (i.e., cell nucleus) [144]. The ability of particles to generate oxygen radicals may also contribute to their carcinogenic potential [227].
With respect to non-malignant respiratory effects, oxidic nickel compounds do not appear to be respiratory sensitizers. Based upon numerous epidemiological studies of nickel-producing workers, nickel alloy workers, and stainless steel workers, there is little indication that exposure to oxidic nickel results in excess mortality from chronic respiratory disease [14, 15, 85-87, 114, 121, 133]. In the few instances where excess risks of non-malignant respiratory disease did appear- for example, in refining workers in Wales- the excesses were seen only in workers with high nickel exposures (> 10 mg Ni/m3), in areas that were reported to be very dusty. With the elimination of these dusty conditions, the risk that existed in these areas seems largely to have disappeared by the 1930s [129].
In a study using radiographs of nickel sinter plant workers exposed to very high levels of oxidic and sulfidic nickel compounds (up to 100 mg Ni/m3), no evidence that oxidic or sulfidic nickel dusts caused a significant fibrotic response in workers was reported [228]. In a study of Norwegian nickel refinery workers, an increased risk of pulmonary fibrosis was found in workers with cumulative exposure to sulfidic and soluble, but not oxidic nickel [20]. The previously mentioned Kilburn et al. [101] and Sobaszek et al. [102] studies (see Section 5.1.1) showed mixed evidence of chronic effects on pulmonary function in stainless steel welders. Broder et al. [229] showed no differences in pulmonary function of nickel smelter workers versus controls in workers examined for short periods of time (1 week); however, there were some indicators of a healthy worker effect in this cohort which may have resulted in the negative findings. Anosmia (loss of smell) has been reported in nickel-cadmium battery workers, but most researchers attribute this to cadmium toxicity [230].
Animal studies have shown various effects on the lung following relatively short periods of exposure to high levels of nickel oxide aerosols [44, 45, 145, 147, 148]. Effects have included increases in lung weights, increases in alveolar macrophages, fibrosis, and enzymatic changes in alveolar macrophages and lavage fluid. Studies of repeated inhalation exposures to nickel oxide (ranging from two to six months) have shown that exposure to nickel oxide may impair particle lung clearance [51]. Chronic exposures to a high-temperature nickel oxide resulted in statistically significant inflammatory changes in lungs of rats and mice at 0.5 mg Ni/m3 and 1.0 mg Ni/m3, respectively [21]. These values correspond to workplace exposures up to 1.6 mg Ni/m3 [43]. At present, the significance of impaired clearance seen in nickel oxide-exposed rats and its relationship to carcinogenicity is unclear [144].
4.5 SULFIDIC NICKEL
Data relevant to characterizing the adverse health effects of nickel "sulfides" in humans arises almost exclusively from processes in the refining of nickel. Exposures in the refining sector should not be confused with those in mining, where the predominant mineral from sulfidic ores is pentlandite [(Ni, Fe)9S8]. Pentlandite is very different from the nickel subsulfides and sulfides found in refining. Although a modest lung cancer excess has been found in some miners [24], this excess has been consistent with that observed for other hard-rock miners of non-nickel ores [231]. This, coupled with the fact that millers have not presented with statistically significant excess respiratory cancer risks, suggests that the lung cancer seen in miners is not pentlandite-related [24]. Pentlandite has not been shown to be carcinogenic in hamsters intratracheally instilled with the mineral over their lifetimes [125], although this study was not conclusive. Therefore, for purposes of this document, any critical health effects discussed relative to “sulfidic nickel” pertains mainly to nickel sulfides (NiS) and subsulfide (Ni3S2).
Like oxidic nickel, inhalation of sulfidic nickel compounds is the route of exposure of greatest toxicological concern in occupational settings. No relevant studies of dermal exposure have been conducted on workers exposed to sulfidic nickel. Because exposures to sulfidic and oxidic nickel compounds have often overlapped in refinery studies, it has sometimes been difficult to separate the effects of these two nickel species from each other. Overwhelming evidence of carcinogenicity from animal studies, however, has resulted in the consistent classification of sulfidic nickel as a "known carcinogen" by many scientific bodies [78, 232-234]; refer to section 5.0 on Hazard Classification below. The evidence is discussed below.
4.5.1 Inhalation Exposure: Sulfidic Nickel
The evidence for the carcinogenicity of sulfidic compounds lies mainly in sinter workers from Canada. These workers were believed to have been exposed to some of the highest concentrations of nickel subsulfide (15-35 mg Ni/m3) found in the producing industry. They exhibited both excess lung and nasal cancers [24, 132]. Unfortunately, as noted in Section 5.4, these workers were also concomitantly exposed to high levels of oxidic nickel, making it difficult to distinguish between the effects caused by these two species of nickel.
Further evidence for the respiratory effects of sulfidic nickel can be gleaned from nickel refinery workers in Clydach, Wales. Specifically, workers involved in cleaning a nickel plant were exposed to some of the highest concentrations of sulfidic nickel at the refinery (18 mg Ni/m3) and demonstrated a high incidence of lung cancer after 15 years or more since their first exposure. Analysis by cumulative exposure showed that Clydach workers with high cumulative exposures to sulfidic nickel and low level exposures to oxidic and soluble nickel exhibited higher lung cancer risks than workers who had low cumulative exposures to all three nickel species combined [24]. Somewhat perplexing, however, was that the risk of developing lung or nasal cancer in this cohort was found primarily in those employed prior to 1930, although estimated levels of exposure to sulfidic nickel were not significantly reduced until 1937. This suggested that other factors (e.g., possible presence of arsenic in sulfuric acid that resulted in contaminated mattes) could have contributed to the cancer risk seen in these early workers [235]. In another cohort of refinery workers in Norway, increased cumulative exposures to sulfidic nickel did not appear to be related to lung cancer risk, although workers in this latter cohort were not believed to be exposed to concentrations of sulfidic nickel greater than about 2 mg Ni/m3 [24].
Because of the difficulty in separating the effects of sulfidic versus oxidic nickel in human studies, researchers have often turned to animal data for further guidance. Here, the data unequivocally point to nickel subsulfide as being carcinogenic. In the chronic inhalation bioassay conducted by the NTP [134], rats and mice were exposed for two years to nickel subsulfide at concentrations as low as 0.11 and 0.44 mg Ni/m3, respectively. These concentrations correspond to approximately 0.5 -6.6 mg Ni/m3 workplace aerosol after accounting for particle size differences and animal to human extrapolation [43, 135, 136]. After two years exposure, there was clear evidence of carcinogenic activity in male and female rats, with a dose-dependent increase in lung tumor response. No evidence of carcinogenic activity was detected in male or female mice. No nasal tumors were detected in rats or mice, but various non malignant lung effects were seen. This study was in agreement with an earlier inhalation study which also showed evidence of carcinogenic activity in rats administered nickel subsulfide [236]. These studies, in conjunction with numerous other studies on nickel subsulfide-although, not all conducted by relevant routes of exposure-show nickel subsulfide to be a potent inducer of tumors in animals [134].
With respect to non-carcinogenic respiratory effects, a number of animal studies have reported on the inflammatory effects of nickel subsulfide on the lung [44, 45, 134, 145, 237, 238]. These have been to both short- and long-term exposures and have included effects such as increased enzymes in lavage fluid, chronic active inflammation, focal alveolar epithelial hyperplasia, macrophage hyperplasia and fibrosis. For sulfidic nickel, the levels at which inflammatory effects in rats are seen are lower than for oxidic nickel, and similar to those required to see effects with nickel sulfate hexahydrate.
The evidence for non-malignant respiratory effects in workers exposed to sulfidic nickel has been mixed. Mortality due to non-malignant respiratory disease has not been observed in Canadian sinter workers [133]. This is in agreement with the radiographic study by Muir et al. [228] that showed that sinter plant workers exposed to very high levels of oxidic and sulfidic nickel compounds did not exhibit significant fibrotic responses in their lungs. In contrast (as noted in section 5.4), excess risks of non-malignant respiratory disease did appear in refining workers in Wales with high exposures to insoluble nickel (> 10 mg Ni/m3). With the elimination of the very dusty conditions that likely brought about such effects, the risk of respiratory disease disappeared by the 1930s in this cohort [129]. In a 2003 study of Norwegian nickel refinery workers, a trend in increased risk of pulmonary fibrosis at the radiological level with cumulative exposure to sulfidic nickel was found [20]. Increased odds ratios were seen at lower cumulative exposures of sulfidic than of soluble nickel compounds. As previously noted, the significance of these results for the clinical diagnosis of fibrosis is not certain.
The mechanism for the carcinogenicity of sulfidic nickel (as well as other nickel compounds) has been discussed by a number of researchers [141-144]. Relative to other nickel compounds, nickel subsulfide may be the most efficient at inducing the heritable changes needed for the cancer process. In vitro, sulfidic nickel compounds have shown a relatively high efficiency at inducing genotoxic effects such as chromosomal aberrations and cell transformation as well as epigenetic effects such as increases in DNA methylation [2]. In vivo, nickel subsulfide is likely to be readily endocytized and dissolved by the target cells resulting in efficient delivery of nickel (II) to the target site within the cell nucleus [239, 240]. In addition, nickel subsulfide has relatively high solubility in biological fluids which could result in the release of the nickel (II) ion resulting in cell toxicity and inflammation. Chronic cell toxicity and inflammation may lead to a proliferation of target cells. Since nickel subsulfide is the nickel compound most likely to induce heritable changes in target cells, proliferation of cells that have been altered by nickel subsulfide may be one of the mechanisms behind the observed carcinogenic effects [144].
Because of these effects, sulfidic nickel compounds appear to present the highest respiratory carcinogenic potential relative to other nickel compounds. The clear evidence of respiratory carcinogenicity in animals administered nickel subsulfide by inhalation, together with mechanistic considerations, indicate that the association of exposures to sulfidic nickel and lung and nasal cancer in humans is likely to be causal [142].
4.6 NICKEL CARBONYL
Unlike other nickel species, nickel tetracarbonyl (commonly referred to as nickel carbonyl) can be found as a gas or as a volatile liquid. It is mainly found as an intermediate in the carbonyl process of refining. By virtue of its toxicokinetics, it is the one nickel compound for which short-term inhalation exposures are the most critical. With respect to dermal exposures, although biologically possible, absorption through the skin has not been demonstrated in humans, nor have any dermal studies on animals been conducted. The discussion, below, therefore, focuses on inhalation exposures.
4.6.1 Inhalation Exposure: Nickel Carbonyl
Nickel carbonyl delivers nickel atoms to the target organ (lung) in a manner that is probably different from that of other nickel species. After nickel carbonyl inhalation, removal of nickel from the lungs occurs by extensive absorption and clearance. The alveolar cells are covered by a phospholipid layer, and it is the lipid solubility of nickel carbonyl vapor that is of importance in its penetration of the alveolar membrane. Extensive absorption of nickel carbonyl after respiratory exposure has been demonstrated. Highest nickel tissue concentrations after inhalation of nickel carbonyl have been found in the lungs, with lower concentrations in the kidneys, liver, and brain. Urinary excretion of nickel increases in direct relationship to exposure to nickel carbonyl [241].
Acute toxicity is of paramount importance in controlling risks associated with exposure to nickel carbonyl. The severe toxic effects of exposure to nickel carbonyl by inhalation have been recognized for many years. The clinical course of nickel carbonyl poisoning involves two stages. The initial stages are characterized by headache, chest pain, weakness, dizziness, nausea, irritability, and a metallic taste in the mouth [242-244]. There is then generally a remission lasting 8-24 hours followed by a second phase characterized by a chemical pneumonitis but with evidence, in severe cases, of cerebral poisoning. Common clinical signs in severe cases include tachypnoea, cyanosis, tachycardia, and hyperemia of the throat [245]. Hematological results include leukocytosis. Chest x-rays in some severe cases are consistent with pulmonary edema or pneumonitis, with elevation of the right hemidiaphragm. Shi [245] reported three patients with ECG changes of toxic myocarditis. The second stage reaches its greatest severity in about four days, but convalescence is often protracted. In ten patients with nickel carbonyl poisoning, there were initial changes in pulmonary function tests consistent with acute interstitial lung disease [244]. However, these results returned to normal after several months.
The mechanism of the toxic action of nickel carbonyl has never been adequately explained, and the literature on the topic is dated [242]. Some researchers have held the view that nickel carbonyl passes through the pulmonary epithelium unchanged [246]. However, as nickel carbonyl is known to be reactive to a wide variety of nitrogen and phosphorous compounds, as well as oxidizing agents, it is not unreasonable to assume that it is probably reactive with biological materials [242]. It is known to inhibit the utilization of adenosine triphosphate (ATP) in liver cells and brain capillaries [247, 248]. Following acute exposure to nickel carbonyl, sections of lung and liver tissue have been shown to contain a granular, brownish-black, noniron-staining pigment [249]. It has not been established, however, whether these dark granules represent metallic nickel or the compound itself. Sunderman et al. [249] proposed that nickel carbonyl may dissociate in the lung to yield metallic nickel and carbon monoxide, each of which may act singly, or in combination with each other, to induce toxicity.
Evidence of chronic effects at levels of exposure below those which produce symptomatic acute toxicity is difficult to find. The only epidemiological study specifically investigating the possible carcinogenic effect of nickel carbonyl [243] was limited in power and confounding factors–such as exposures to certain oxidic and sulfidic nickel species–thereby clouding any interpretation regarding the contribution of nickel carbonyl, per se, to the carcinogenic risk.
Like humans, the lung is the primary target organ from exposure to nickel carbonyl in animals, regardless of route of administration, and effects in animals are similar to those observed in cases of human exposure. Experimental nickel carbonyl poisoning in animals has shown that the most severe pathological reactions are in the lungs with effects in brain and adrenal glands as well. Acute toxicity is of greatest concern. The LD50 in rats is 0.20 mg Ni/liter of air for 15 minutes or 0.12 mg/rat. Effects on the lung include severe pulmonary inflammation, alveolar cell hyperplasia and hypertrophy, and foci of adenomatous change.
With respect to carcinogenic effects, studies on the carcinogenicity of nickel carbonyl were performed prior to present day standardized testing protocols, but because of the extreme toxicity of this material, further studies are not likely to be conducted. Studies by Sunderman et al., [249] and Sunderman and Donnelly [250] have linked nickel carbonyl to respiratory cancer, but high rates of early mortality in these studies preclude any definitive conclusions regarding the carcinogenicity of nickel carbonyl. Possible developmental toxicity effects are also of concern for nickel carbonyl. In a series of studies, Sunderman et al. [251, 252] demonstrated that nickel carbonyl, administered by inhalation (160-300 mg Ni/m3) or injection (before or a few days after implantation) produced various types of fetal malformations in hamsters and rats.
[1] Forced Expiratory Volume (FEV1) is the amount of air that you can forcibly blow out in one second, measured in litres. Forced vital capacity (FVC) is the amount of air that can be maximally forced out of the lungs after a maximal inspiration. The FEV to FVC ratio reflects the severity of pulmonary impairment in obstruction (healthy adults should be between 75-89%).